FiberSense technology
Continuous Glucose Monitoring
Continuous blood glucose monitoring offers reliability and comfort for patients. The risk of dangerous hypoglycaemia attacks is reduced, along with potential late-onset diabetic complications caused by high blood glucose values. Health insurance providers began including these devices in their range of coverage in 2016.
The FibreSense system from EyeSense GmbH does not measure glucose in the blood, but rather painlessly and reliably in the subcutaneous interstitial fluid. A miniature fiber optic sensor, worn on the arm or abdomen, sends the monitored glucose value to the patient’s smartphone or read-out device wirelessly.
On his smartphone or read-out device, the patient can see the current value, as well as the trend (rising/falling) and a chronological chart tracing the values measured last.
Concept
FiberSense technology is based on an optical glucose sensor which measures the glucose concentration of the tissue fluid at the tip of an optical fiber. This level of concentration very closely correlates to blood glucose levels. The sensor material consists of a biosensor that reacts specifically to glucose. It is embedded in an aqueous hydrogel at the tip of a thin light-transmitting fiber. This is implanted a few millimeters under the skin. The continuous monitoring is conducted via a miniature fluorescence photometer which is held onto the skin with a long-term adhesive bandage.
The readings are sent wirelessly to a read-out device or smartphone. On the display, the user can see the current glucose values, set alarms or follow his blood glucose curve graphically.
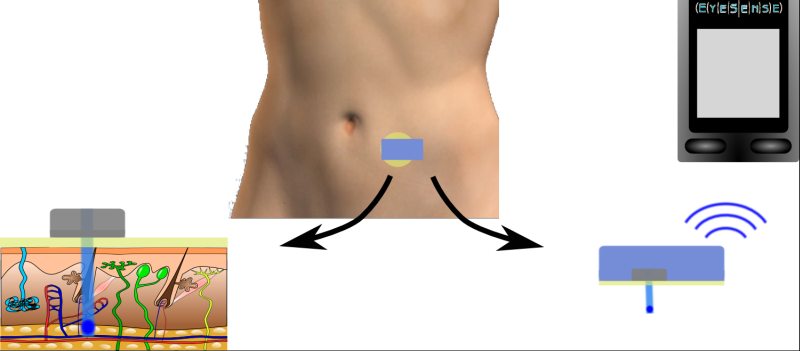
Figure 1. Diagram of FiberSense System. Below left: Cross-section of skin with optical fiber (light blue) with sensor (dark blue) at its tip as well as adhesive bandage and fluorescence photometer on the patient’s skin. Below right: FiberSense system with optical fiber and sensor, adhesive bandage, and fluorescence photometer (blue).
Technology
The glucose sensor technology developed by EyeSense is based on a receptor molecule capable of specifically binding to glucose, and a competitor molecule which competes with glucose at the binding site of the receptor molecule. The receptor molecule builds complexes with glucose as well as with the competitor molecule. By labeling the receptor and competitor molecules with differing fluorescent dyes, the formation of complexes becomes measurable. Fluorescent dyes are dyes which when exposed to light of a particular color; emit light of a differing color.
One of the chosen fluorescence dyes, the energy donor, can transfer excitation energy to the other fluorescence dye, or energy acceptor, if they are close to each other. This transfer leads to diminished fluorescence on the part of the donor dye. This only occurs, however, when the receptor and competitor molecule build a complex. If the competitor molecule is displaced by glucose, no energy can be transferred from the donor to the acceptor, and hence the donor fluorescence increases in intensity. The intensity of the donor fluorescence is therefore the measuring factor for glucose concentration.
The glucose sensor is embedded in an aqueous hydrogel. A miniscule amount is applied on the tip of a very thin optical plastic fiber which is inserted a few millimeters subcutaneously and measures the glucose concentration in the interstitial fluid. Values are read via a sensitive fiber optic.
Mobil App
We have developed a special read-out device in smartphone format for displaying blood glucose values. Patients who would like to avoid having too many devices can use the monitoring system with their smartphone. We have developed an app for this (currently iOS and selected Android operating systems). The app can display blood glucose levels, blood glucose curves, trends and sensor status. It is possible to set alarms individually as well as threshold values. The diary function makes it possible to enter carbohydrates, insulin doses and activities. It is also possible to trace alarms over time. Measurement data and diary entries can then be copied onto a computer for further processing.
Clinical Results
Clinical feasibility study for a transdermal, continuously operating fiber optic sensor
Introduction
Currently available Glucose Monitoring Systems (CGMS) have a short life expectancy. In this study, a new CGM system, FiberSense, was tested. It is based on a fluorescing biosensor at the tip of an optical fiber.
Study protocol
The clinical trials were conducted over a 28-day period at the Diabetes Institute in St Joseph’s Hospital in Heidelberg, Germany. FiberSense was placed in the subcutaneous tissue in the abdomen or upper arm in both Type 1 and Type 2 diabetic patients. Data was collected over several monitoring sessions. The patients were given glucose stimulation (orally administered glucose and delayed insulin administration). The FiberSense data was then compared to capillary blood measurements (standard laboratory method) as well as to those of a commercially available CGM system implanted on the other side of the abdomen (for up to 7 days). In addition, some monitoring sessions took place while patients conducted their normal daily activities for a few hours.
Results
During the 28-day research period, the FiberSense data remained within a range of error of 20% (Requirement of ISO 15197*) compared to the laboratory data. It was much more accurate than the commercially available CGM system.
*Source: ISO 15197. In vitro diagnostic test systems: requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus. International Organization for Standardization, 2003.
In general, the Mean Absolute Relative Deviation (MARD) lay between 8 and 9 percent depending on the location of the sensor; compared to 18 percent for the commercially available CGM System. 93 percent of the measurements were, compared with laboratory data, in the range of correct clinical treatment decisions (consensus error grid zones A and B†)
† Source: Parkes et al., A new consensus error grid to evaluate the clinical significance of inaccuracies in the measurement of blood glucose, Diabetes Care 23: 1143-1148, 2000.
Conclusions
The FiberSense CGM system, with its fiber optic sensor, displays excellent precision over a time period of four weeks. This is a considerable improvement on currently available systems which can only monitor for one week at most.
If you are interested in further information, we look forward to hearing from you at EyeSense.
Publications
The detailed study results have been presented at several international conferences and published in scientific journals.
Müller et al., Clinical Feasibility Study of a Percutaneous Optical Glucose Fiber Sensor, Nov 2012, DTM, San Francisco.
Müller et al., Clinical Feasibility Study of a Percutaneous Optical Glucose Fiber Sensor, Feb 2013, ATTD, Barcelona.
Müller et al., First Clinical Evaluation of a New Percutaneous Optical Fiber Glucose Sensor for Continuous Glucose Monitoring in Diabetes, J Diabetes Sci Technol, 2013; 7(1): 13-23.


